We are delighted to extend a warm welcome to the newest centres joining our network:
- Federal University of Rio de Janeiro, Brazil
- Hospital Infantil de México Federico Gómez, Ciudad de México
- Benemérito Hospital General Juan María de Salvatierra, La Paz, BCS, México
- Hospital Roberto del Río, Santiago de Chile
As usual, all newly enrolled centres are undergoing ethics approval before beginning patient recruitment and will sign individual Material Transfer Agreements (MTAs) before the first biomaterial shipment.
The consortium now proudly includes over 70 centres across 36 cities in 18 LATAM countries. You can check them HERE. If you know of any centres that have not yet joined, we would love to hear from them, feel free to connect them with us!
The process for exporting biological samples to Newcastle is well established with most countries and samples arriving smoothly. However, three countries -Argentina, Chile, and Mexico- currently face challenges due to certification requirements. In Mexico, we have resolved this by partnering with an external laboratory that holds the necessary certifications. In Argentina, two centres have obtained its own certification and will soon ship over 100 samples. For Chile, we are actively exploring possible solutions.
Additionally, El Salvador is experiencing challenges with receiving saliva collection kits, which are classified as medical devices. We are in the process of securing the required certification, after which sample collection can begin as we work to understand the requirements for exporting their samples.
Thanks to all efforts, since September 2023, we have received over 750 biological samples from 17 centres in 11 countries (Fig.1), in various formats such as DNA, fresh blood, dry blood, and saliva. (Fig.2). These samples consist of 58% from affected patients and 42% from unaffected family members (Fig.3). The majority of samples are from paediatric and young adult patients affected by neuromuscular diseases (Fig.4).
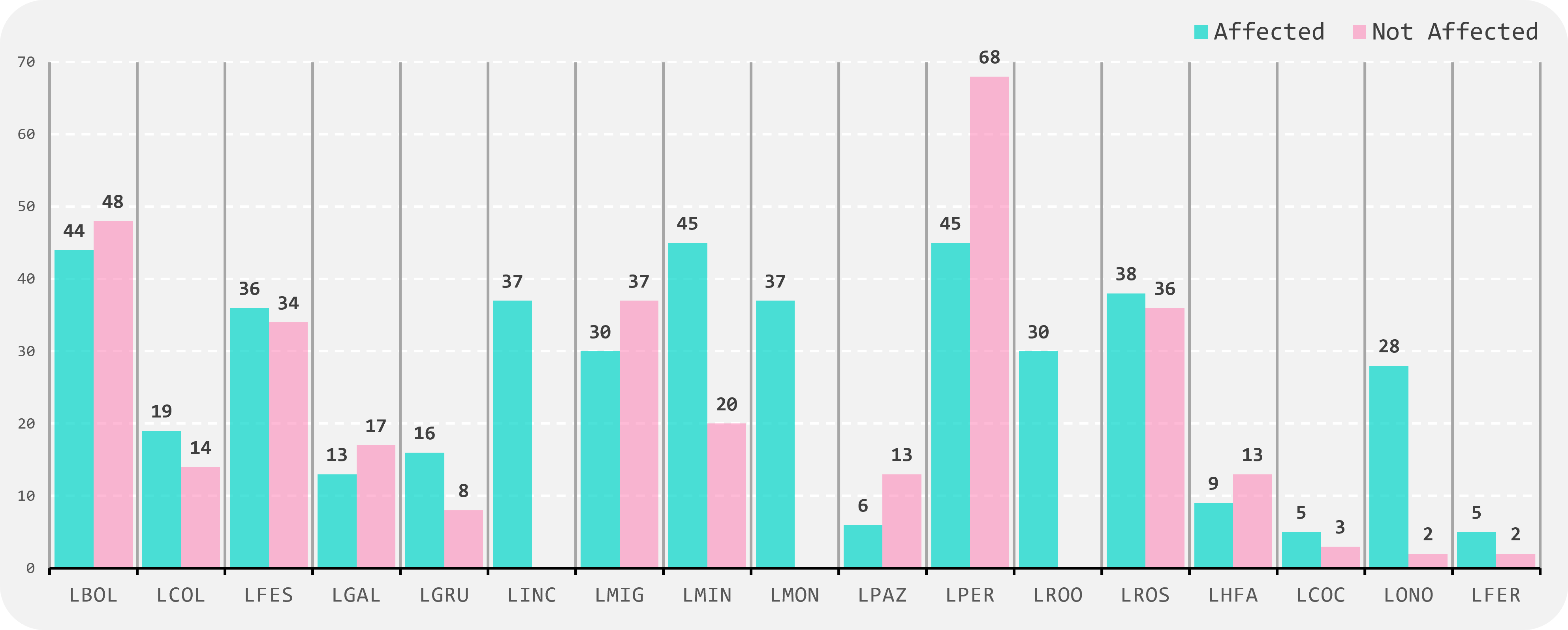
FIGURE 1
Number of samples collected from Affected patients and Not-Affected relatives, from 17 centres in 11 countries.
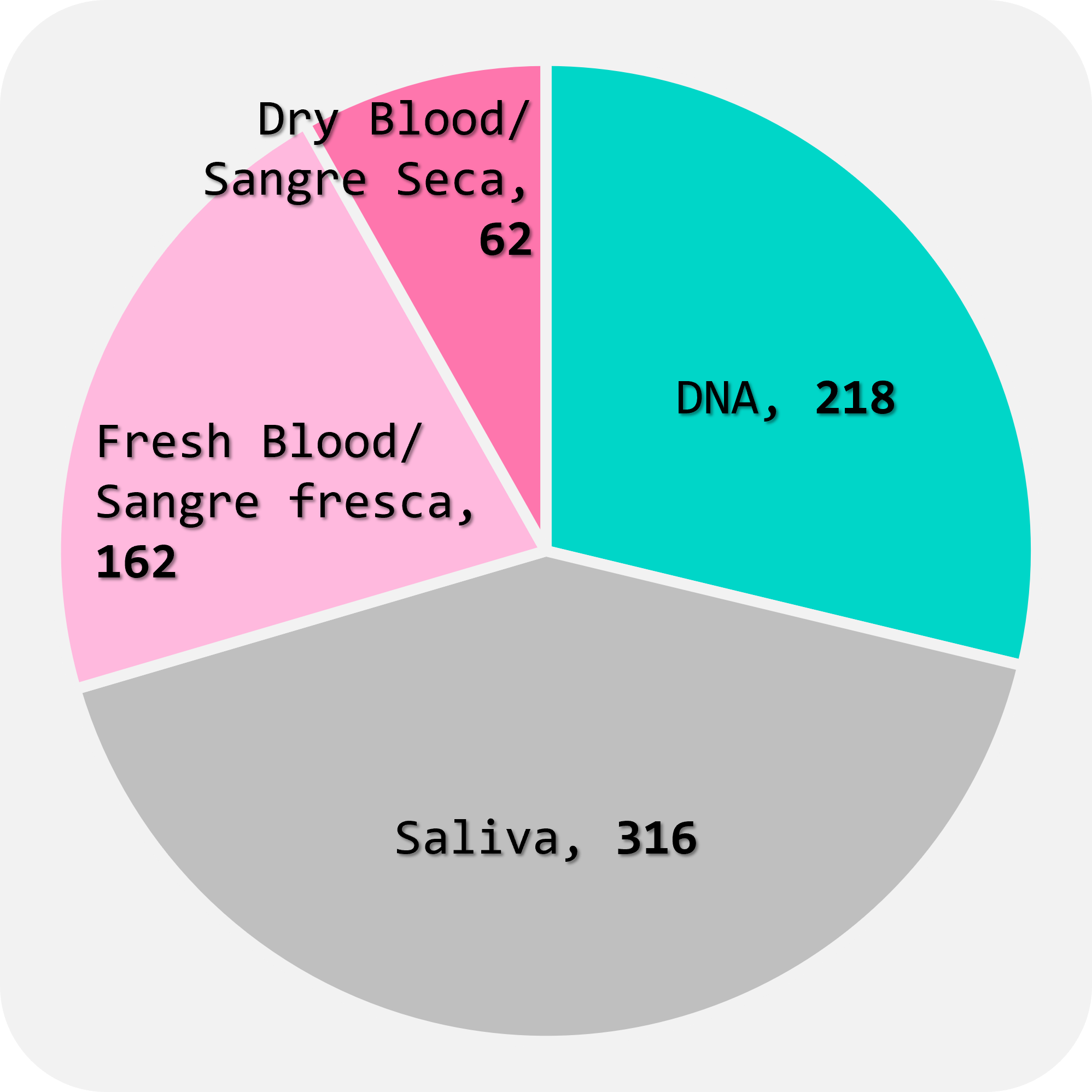
FIGURE 2
Tipes of samples received so far (758)
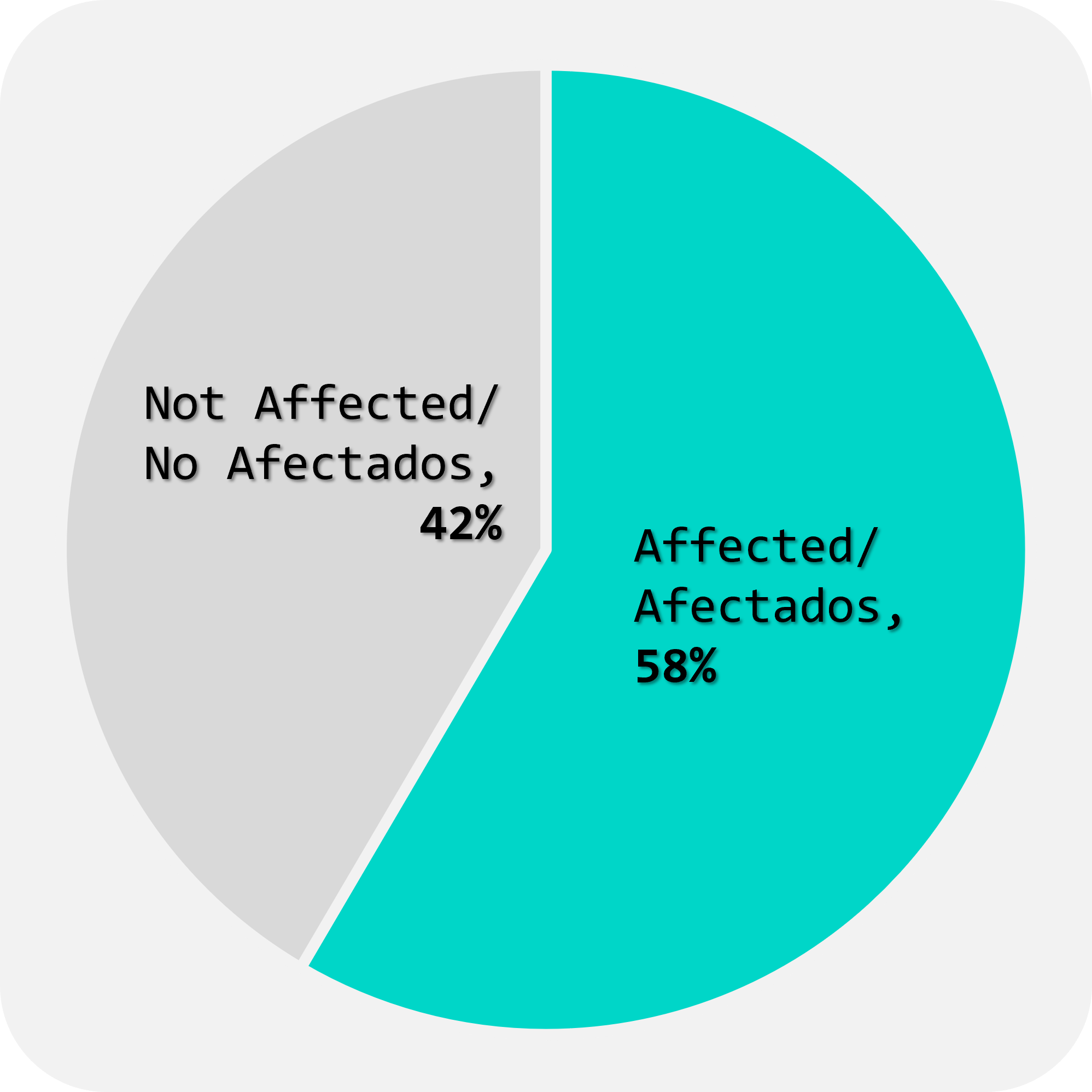
FIGURE 3
% of samples received from Affected patients and Not-Affected relatives.
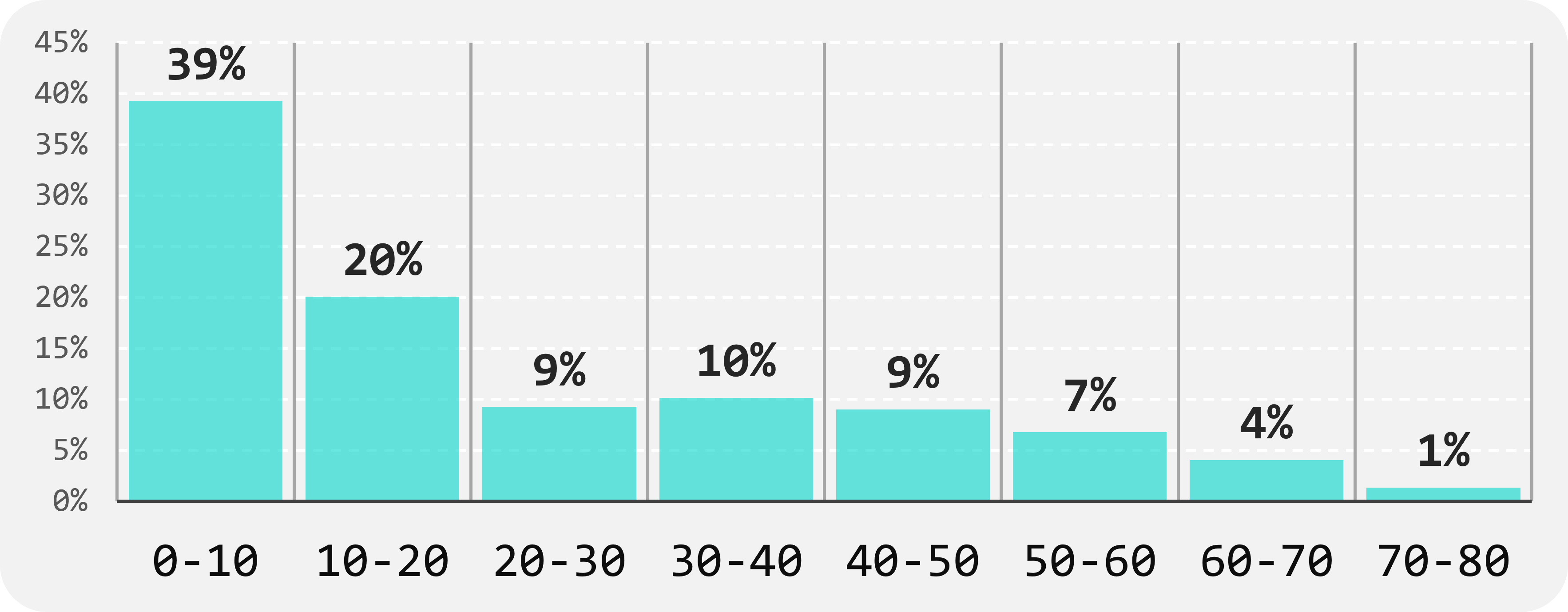
FIGURE 4
Age ranges (X) of patients (affected only) received so far (Y)
The inclusion criteria specify that patients must have a neuromuscular disease expected to be genetic. The current breakdown by clinical suspicion is shown in Fig.5.
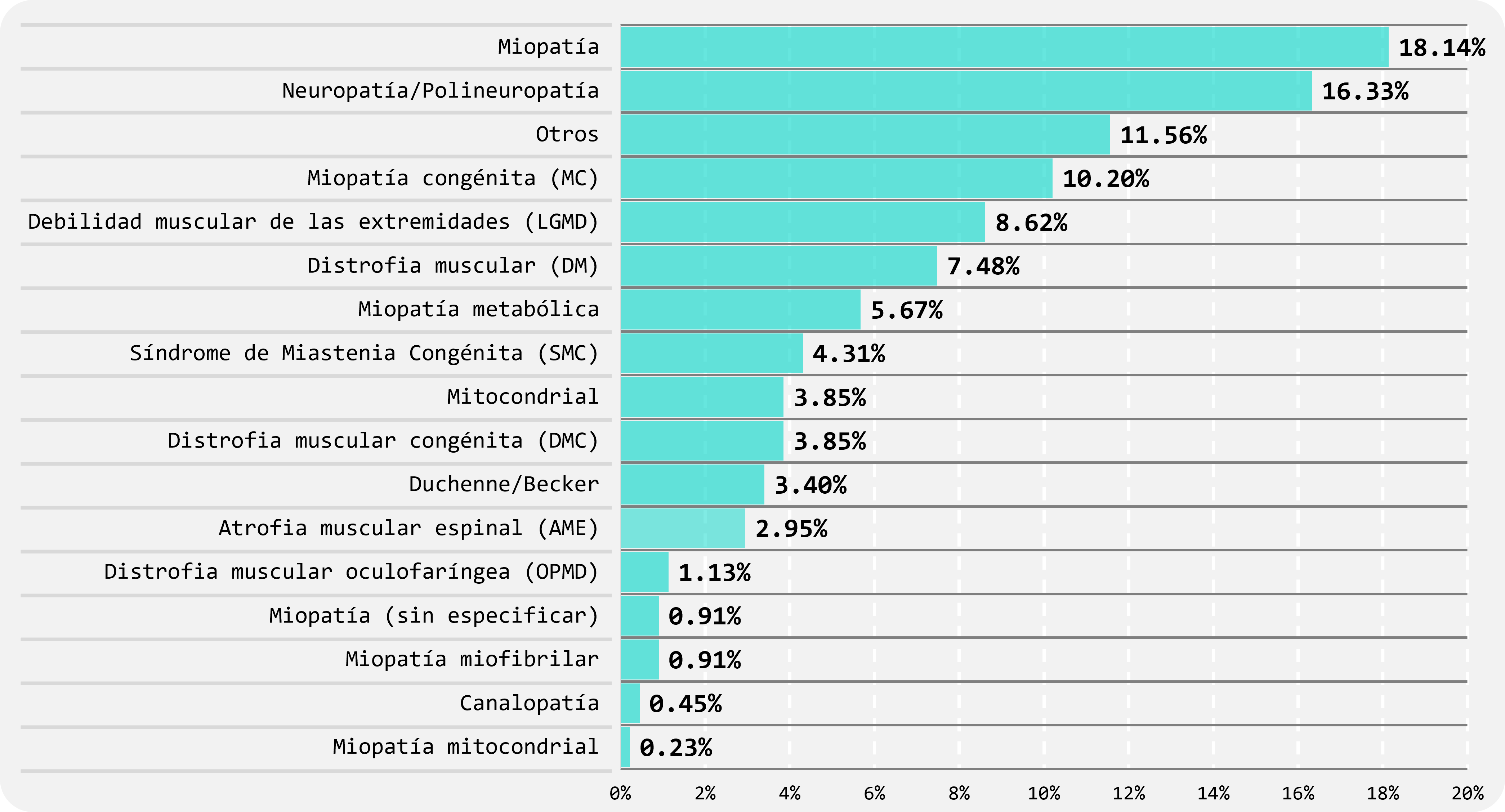
FIGURE 5
Clinical suspicion of patients (affected only) received.
Single gene pre-screening:
A total of 80 samples has been subjected to Sanger sequencing point mutation analysis or MLPA to detect deletions or duplications in specific genes of interest. An overall solved rate of 33.75% has been achieved by this approach, with 44% Charcot-Marie Tooth and 61.5% for SMA diagnostic yield. The cases that remained negative after this single gene pre-screening have been sent for Whole Exome Sequencing (WES). (See Fig.6)
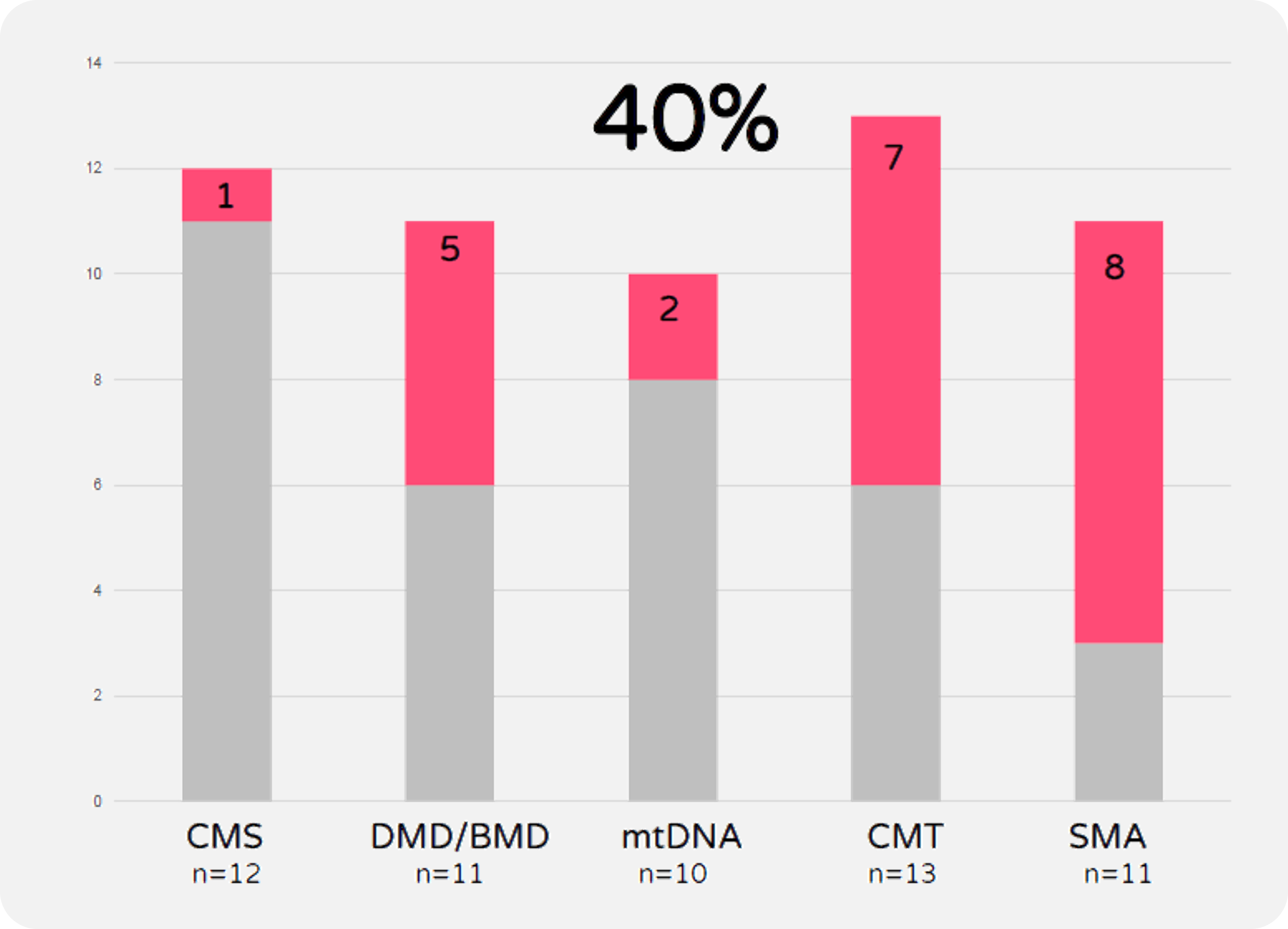
FIGURE 6
Phenotype-driven pre-screening solved rate for patients with clinical suspicion of Congenital Myasthenic Syndrome (CMS), Duchenne & Becker Muscular Dystrophy (DMD/BMD), mitochondrial myopathy (mtDNA), Charcot-Marie-Tooth 1A (CMT) and Spinal Muscular Atrophy (SMA).
Whole exome sequencing (WES):
Proband-only exomes have been fully analysed for single nucleotide variants (SNVs) and indels using the RD-connect platform GPAP (https://rd-connect.eu/). Standard filtering criteria for rare diseases have been implemented, including minor allele frequency (MAF) <1% and high to moderate variant effect predictor (VEP) and applying a gene list of 674 genes known to be associated with muscle conditions. ACMG guidelines were followed for variant pathogenicity classification. Pathogenic and likely pathogenic variants as well as variants of unknown significance identified in each patient were then discussed with the referring clinician in multidisciplinary meetings (MDTs). Clinical presentations were reevaluated together with family history, histopathology, and MRI when available in order to confirm or rule out uncertain findings. Based on this, genetic findings are classified into five categories: positive, compatible, uncertain, incompatible, and negative. This approach is of particular importance when single heterozygous pathogenic variants in recessive genes (ie compatible) or findings in non-relevant genes (i.e. incompatible) are identified as it guides further genetic and/or clinical tests.
 |
Positive: A pathogenic or likely pathogenic variant in a gene matching the clinical presentation of the patient |
 |
Compatible: a) A variant of unknown significance in a gene matching the clinical presentation and mode of inheritance or b) a single pathogenic or likely pathogenic variant in a recessive gene strongly matching the clinical presentation of the patient. |
 |
Uncertain: A variant of unknown significance in a gene compatible with the clinical presentation of the patient |
 |
Incompatible: A pathogenic or likely pathogenic variant in a gene not matching the clinical presentation of the patient. |
 |
Negative: No relevant variants identified amongst the in-silico gene list analysed. |
The overall diagnostic rate so far (n=42) is 43%, including positive and compatible findings. A large proportion of genes identified are causative of neuropathies, followed by congenital myopathies, and only two muscular dystrophies, in line with the clinical suspicion stated by the referring clinician. The remaining 57% includes negative, uncertain, and incompatible cases, which will be further evaluated in second round of analyses (Fig.7). Another 40 exome datasets are currently being interpreted, and a CNV pipeline has been implemented and data analysis ongoing. In addition, 150 DNA samples are undergoing library preparation at the sequencing provider (CNAG) and are expected to be completed before the end of the year.
Interestingly, even at this very early stage in the project, a novel candidate gene not yet associated with muscle conditions has already been identified. As a result, the patient, from a participating centre in Lima, Peru, has been included in a recently submitted publication, which will help highlighting the potential significance of this gene in the context of muscle-related diseases.
Each case recruited into the study receives a report where the genetic findings are detailed. If positive, reports include gene and variant identified, predicted effect at the protein level, population frequency, links to previous publications and OMIM entries, ACMG classification and technical details. Case-specific recommendations, such as segregation studies and functional tests are provided. Patients are also signposted to patients’ organizations, registries, and clinical trials, if available. Genetic reports were issued and provided to the referring doctors, who are responsible for communicating this information to the patients and their families. These reports have been created in a unique tailored patient-centred manner in the context of multidisciplinary discussions.
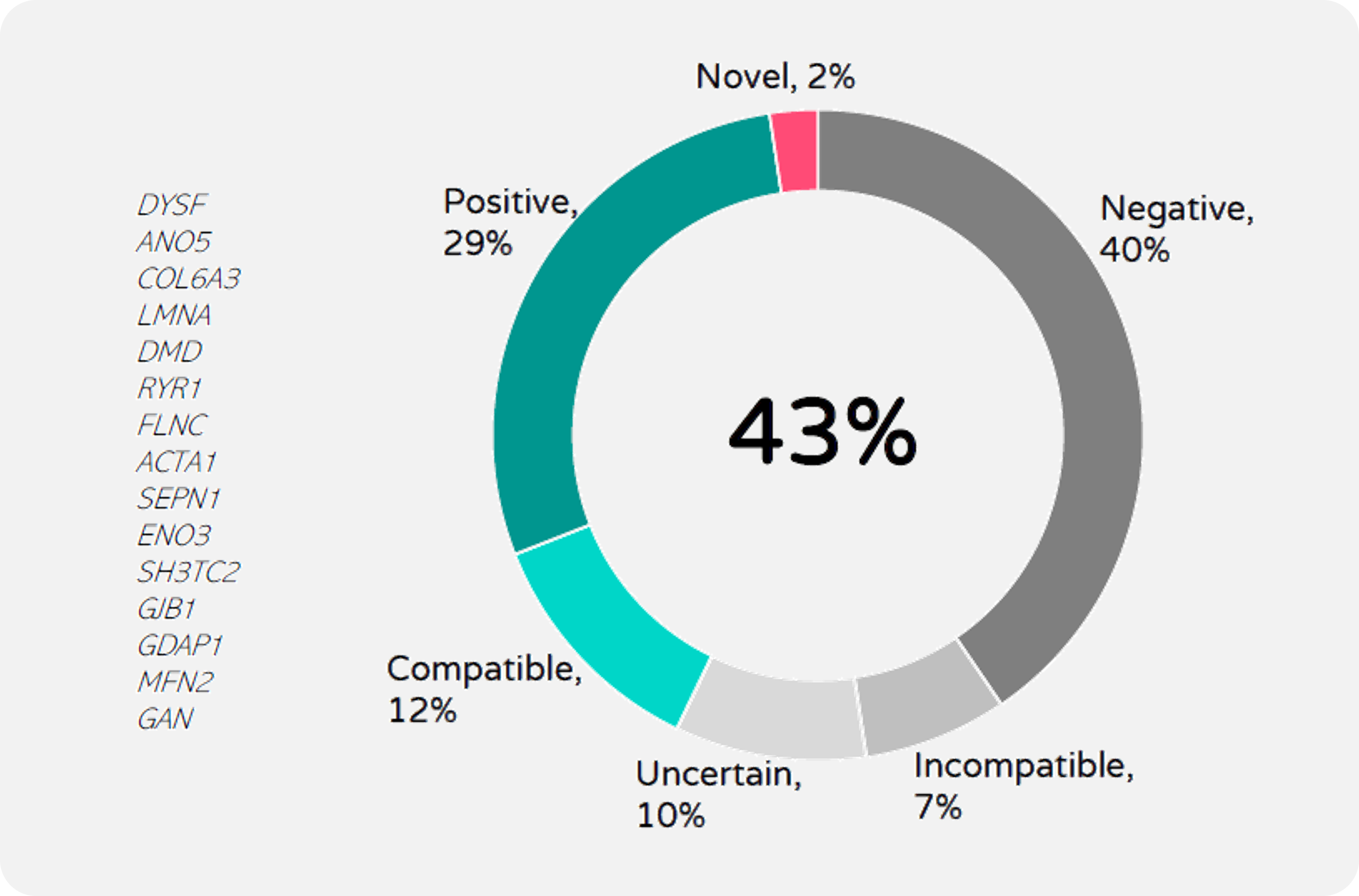
FIGURE 7
Exome-based solved rate (n=42). Genetic reports were divided into: Positive: P/LP variant(s) identified in genes that match phenotype and mode of inheritance; Compatible: a single P/LP variant identified in a recessive gene that matches the clinical presentation; Uncertain: variants of unknown significance; Incompatible: P/LP variant(s) identified in genes not compatible with the clinical presentation; Negative: no relevant causative variants found among the 672 NMD genes analysed. Genes identified are listed (all n=1, except FLNC n=2)
To date, we have delivered four seminars and held one collaborative discussion on resolved Latin-SEQ cases.
On the 17th of December 2024 we will do a review and closure of the year.
We are designing the 2025´s calendar, starting in January with a collaborative discussion of solved cases from “Neuromusculares Peru”, and following with some seminars including topics like “Mitochondrial variant analysis and interpretation at exomes”, “ATEND: development of an assessment for patients with severe involvement in SMA” and “Exome Analysis of Neuromuscular Patients”.
We encourage everyone to present their work or any topics of interest for the consortium during these sessions. To book a date, please reach out to us. If you don’t have access to the session recordings yet or would like to join future ones, feel free to get in touch.




Latin-SEQ has also secured funding to support four work placements for LATAM clinicians at the JWMDRC in Newcastle or at the Sant Joan de Déu Barcelona Hospital. To date, two clinicians have taken advantage of this opportunity, completing two-month placements at the JWMDRC. These placements have provided hands-on experience in genetics, laboratory work, and clinical patient care, at no cost to them. In 2025, we look forward to hosting two more clinicians, empowering them to advance their careers and contribute to the LATAM healthcare community.
Stablishing a Steering Committee was a key milestone, enabling structured oversight and decision-making for our project. Steering Committees are integral in guiding complex, multi-national research projects like ours, as they bring together a range of expertise to provide strategic direction, ensure compliance with ethical standards, and facilitate effective communication across teams and regions.
You can view the members of the committee HERE.
The Committee meets quarterly to review progress, assess outcomes, and plan next steps. Key documents, including the “Terms of Reference”, “Publication Policy”, “Data Access and Sharing Agreement”, and “Patient Consent for Treatment Access”, are being developed and will be shared with the consortium in due course.
This committee helps align the project with international standards, fostering a unified vision across LATAM and supporting our impact in the neuromuscular disease field.
The Latin-SEQ project has been presented and actively promoted at various local, national, and international conferences (see list below), where it has generated significant interest and collaborative opportunities. By presenting the project’s objectives, methodologies, and initial findings, the Latin-SEQ team has engaged with researchers and clinicians interested in expanding genetic research and diagnostic capabilities in LATAM. The project’s presence at these events has been instrumental in gathering support, fostering partnerships, and encouraging wider participation, which is critical for achieving the project’s aims of enhancing genetic diagnostic access and understanding neuromuscular diseases within the region.
In addition to its diagnostic and education components, the Latin-SEQ consortium actively promotes research projects in both clinical and genetic fields. These projects include, but are not limited to, phenotype-genotype correlation analysis, description of founder variants, identification of new genes, and the study of genetic factors specific to the Latin American region. Each participating centre is free to utilise the results generated according to its own interests and objectives.
Furthermore, the consortium encourages members to engage in conferences and contribute to scientific publications. So far, an abstract led by the Instituto Nacional de Rehabilitacion, a participating centre from Mexico has been submitted to a local journal. An updated list of all ongoing projects, publications, and presentations will also be available in the intranet section of this Latin-SEQ website.
To ensure fair and balanced participation in these activities, a publication policy is in place, which will be available on the website’s intranet or can be requested directly from us. The Latin-SEQ project values the contribution and effort of all participating centres and collaborators. To guarantee fair and collaborative representation, any overarching publication made by the coordinating team in Newcastle will include the Latin-SEQ Consortium members who have contributed samples to the study. This inclusion ensures appropriate recognition of all participants and promotes visibility of the collective work within the scientific community. Additionally, each centre retains full freedom to lead local work and/or presentations of individual cases. This publication policy fosters transparency, international collaboration, and scientific advancement, allowing the findings and knowledge generated through the Latin-SEQ project to be widely shared, benefiting the medical and scientific community as well as patients with neuromuscular diseases.
Presentations:
| DATE | PRESENTATION (Oral Or Poster) | LOCATION |
| Jun 2023 | Solane 23 (Sociedad Latinoamericana de Enfermedades Neuromusculares) | Bogota, Colombia |
| Oct 2023 | WMS 23 (World Muscle Society) | Charleston, USA |
| Mar 2024 | CLACS 24 (Centre for Latin American and Caribbean Studies) | Newcastle, UK |
| Mar 2024 | JAIN Conference 2024 | Houston, USA |
| Jun 2024 | Australian Genomics’ DNA dialogue seminar | Online |
| Oct 2024 | WMS 24 (World Muscle Society) | Prague, Czech |
| Nov 2024 | XI ICRR (International Congress of Rehabilitation Research) | Ibarra, México |
| Nov 2024 | EVELAM 24 (Escuela de Verano Eurolatinoamericana de Miología) | Cuzco, Perú |
| May 2025 | Solane 25 (Sociedad Latinoamericana de Enfermedades Neuromusculares) | México City |
Publications:
- Vulto-van Silfhout et al. Loss-of-function variants in… [confidential information]. Submitted to the European Journal of Human Genetics. October 2024
- Novas Maldonado et al. Whole exome sequencing analysis of 1000 Latin American patients affected by a neuromuscular disorder. Preliminary data. 2024. Submitted to Investigación en Discapacidad, México.
The Latin-SEQ project is proving to be a success, and we hope you share this sentiment. We deeply appreciate the contributions of all our collaborators, whose efforts have been instrumental in expanding and solidifying the consortium, as well as the patients and families who have participated. Thank you!
By supporting a strong network across the LATAM continent, Latin-SEQ aims to advance diagnostic capabilities and healthcare knowledge in neuromuscular genetics. Our goal is to provide a virtual space that allows neurologists and clinicians to connect and collaborate, making rare diseases less rare through shared expertise and resources.
While we are seeing significant progress, including a high diagnostic yield and new gene discoveries, there has been a slight delay in delivering genetic reports for the recruited patients. We appreciate your patience as we work to resolve this, including the addition of extra support for data analysis. We are confident that once this is in place, the process will run more smoothly.
We are also exploring new initiatives to further support the neuromuscular communities in LATAM and are working on providing training materials through this website. Your feedback is invaluable, and we welcome any suggestions for improving Latin-SEQ.
Our vision is to sustain and expand Latin-SEQ over time, ensuring that its benefits are scaled to improve patient outcomes and healthcare infrastructure across LATAM. Any support in raising visibility or securing financial backing is greatly appreciated.